Bronze, Brass, Nickel Silver and Copper Base Metals
Many of us have turned to base metal wires when precious metal prices soured. So what are base metals, anyway?
What are Base Metals?
A chemistry textbook would tell you (and I was surprised to discover this) that a base metal is a metal that oxidizes or corrodes relatively easily, and reacts with hydrochloric acid to form hydrogen. This includes metals such as iron, lead, nickel, copper (although it doesn't do the hydrogen trick) and zinc. The term "base" comes from contrasting the metals with "noble" or "precious" metals, as set by alchemists!
The U.S. Customs and Border Protection has a standard list of base metals, including iron, steel, copper, lead, nickel, zinc, aluminum, tin, titanium, and several other elements excluding gold, silver, and platinum.
The U.S. Customs and Border Protection has a standard list of base metals, including iron, steel, copper, lead, nickel, zinc, aluminum, tin, titanium, and several other elements excluding gold, silver, and platinum.
COPPER
Here's an interesting statistic about copper I found: although copper has been mined and used for over 10,000 years, more than 95% of all copper ever mined and smelted, has been extracted since 1900 - that's in the last 112 years! However, I found that an estimated 80% of extracted copper is still in use today, whether still part of structures or being recycled (copper keeps the same level of quality when recycled as when it is first smelted).
Copper is extremely soft; it only rates a 2.5-3 on Mohs scale! (No wonder it is a good practice wire: if you can wrap a cab in copper wire with no tool marks, you are getting good!) Its elemental symbol is Cu, which comes from cuprum; this is short for cyprium, because the Romans sourced all their copper from the island of Cyprus. You may already know that when copper corrodes, like copper roofs or statues, it gets a green coating called verdigris - but did you know that that same property of copper is how azurite, malachite, and turquoise get their vibrant green and green-blue colors? That's right, both these stones are exposed to copper as they lie in the earth, imparting those beautiful colors.
Copper is extremely useful in wire jewelry: you can create an entire cabochon frame, not like it, cut it apart, and only be out a few cents in materials. Plus, you can save your scrap and take it to a commercial recycler (the type that takes aluminum cans) every year or so. Strike up a conversation with your electrician, contractor, or handyman and you'll find that copper prices have gone up for them just like silver has gone up for us: steeply over the last couple decades - but it is still a bargain for us compared to silver!
Copper is mined all over the world, with 1/3 of the world's copper mined from large sources in Utah and New Mexico in the US, Chile, Indonesia, and Peru. In Sweden, miners operated the Great Copper Mountain from the 900s to 1992 (over 1000 years!): this allowed Sweden to have a copper-backed currency (imagine, a "Copper Standard"!) and in the 1600s, supplied 2/3 of European demand for copper.
Copper is extremely soft; it only rates a 2.5-3 on Mohs scale! (No wonder it is a good practice wire: if you can wrap a cab in copper wire with no tool marks, you are getting good!) Its elemental symbol is Cu, which comes from cuprum; this is short for cyprium, because the Romans sourced all their copper from the island of Cyprus. You may already know that when copper corrodes, like copper roofs or statues, it gets a green coating called verdigris - but did you know that that same property of copper is how azurite, malachite, and turquoise get their vibrant green and green-blue colors? That's right, both these stones are exposed to copper as they lie in the earth, imparting those beautiful colors.
Copper is extremely useful in wire jewelry: you can create an entire cabochon frame, not like it, cut it apart, and only be out a few cents in materials. Plus, you can save your scrap and take it to a commercial recycler (the type that takes aluminum cans) every year or so. Strike up a conversation with your electrician, contractor, or handyman and you'll find that copper prices have gone up for them just like silver has gone up for us: steeply over the last couple decades - but it is still a bargain for us compared to silver!
Copper is mined all over the world, with 1/3 of the world's copper mined from large sources in Utah and New Mexico in the US, Chile, Indonesia, and Peru. In Sweden, miners operated the Great Copper Mountain from the 900s to 1992 (over 1000 years!): this allowed Sweden to have a copper-backed currency (imagine, a "Copper Standard"!) and in the 1600s, supplied 2/3 of European demand for copper.
RECYCLED COPPER
Copper doesn't have to be smelted to be recycled: you can simply reuse the copper wire found in house wiring and appliances. Now, can you seriously use stripped electrical copper wire in jewelry? Well, firstly, electrical wire is only going to be round, and may come in a size you aren't expecting (a fat piece of wire may actually contain dozens of strands of 30-gauge or thinner wire, or several large-gauge wires). So if you enjoy creating traditional wire jewelry with square and half round wire, or you like consistency, stick with copper wire that is sold for making jewelry.
Electrical copper wire will be about 99% pure, just like copper jewelry wire, since it needs to be as pure as possible to conduct electricity effectively. Some people might be concerned if the copper has been exposed to lead wire coverings, but I haven't found any information that says it is a real concern.
However, there are several considerations when recycling electrical wire. First, the temper will probably be dead soft, which means unless you work-harden your pieces well in a tumbler or with a hammer, the resulting piece could be very malleable and could deform. Of course, if you're used to working with dead soft copper wire, you already anticipate this.
The electrical copper wire also is not manufactured with appearance in mind. You might find dirt or burn marks on the wire, which will you will want to clean off with steel wool. Also, the wire may have been milled with rough patches (less common in modern wire, but if you are stripping wire from an old house, you might find this). Also, if you are stripping the copper wire from the plastic tubing yourself, be careful be to slow and deliberate so as not to ding, kink, or damage the wire.
For me, the effort doesn't seem worth it - but if someone gave me a truckload of old electrical wire, I would probably take a stab at it!
Electrical copper wire will be about 99% pure, just like copper jewelry wire, since it needs to be as pure as possible to conduct electricity effectively. Some people might be concerned if the copper has been exposed to lead wire coverings, but I haven't found any information that says it is a real concern.
However, there are several considerations when recycling electrical wire. First, the temper will probably be dead soft, which means unless you work-harden your pieces well in a tumbler or with a hammer, the resulting piece could be very malleable and could deform. Of course, if you're used to working with dead soft copper wire, you already anticipate this.
The electrical copper wire also is not manufactured with appearance in mind. You might find dirt or burn marks on the wire, which will you will want to clean off with steel wool. Also, the wire may have been milled with rough patches (less common in modern wire, but if you are stripping wire from an old house, you might find this). Also, if you are stripping the copper wire from the plastic tubing yourself, be careful be to slow and deliberate so as not to ding, kink, or damage the wire.
For me, the effort doesn't seem worth it - but if someone gave me a truckload of old electrical wire, I would probably take a stab at it!
NICKLE SILVER
Nickel Silver, known by a host of other names including German Silver, albata, new silver, and alpaca/alpacca, is an alloy of nickel, zinc, and copper. Because it's alloyed with zinc and copper, some people regard it as a relative of brass. It's important to note (and tell your customers) that nickel silver doesn't actually contain any silver; instead, it is engineered to closely resemble silver at a fraction of the cost.
Nickel silver was developed, as an alternative to the Chinese alloy paktong, by German metalworkers in the 19th century. The Chinese developed this silver look-alike centuries ago, with secret exports to Southeast Asia resulting in an effort by colonizing Europeans to duplicate the guarded formula. In the 1700s metalworkers got close, and in 1823, German competition yielded a winner. Around the same time, a British man discovered a similar metal alloy.
Following its discovery, the use of nickel silver exploded. It has been used in on dining tables nearly since its discovery; some sets of silverware are stamped EPNS, which stands for Electro-plated nickel silver, which is silverware that has been made with nickel silver and electroplated with real silver. These "silver" sets are of no value to a metal recycler, but are becoming more rare every year, so perhaps they will have historical value. Also, as the silver finish wears away, the nickel silver is actually brighter, due to its tarnish resistance, so an EPNS set Used practically everywhere: keys, zippers, jewelry, silverware, wind instruments including French horns and flutes (can be silver-plated) and frets of guitars, automobiles, coins and even tracks in model railroads. It typically resists tarnish and corrosion. Used extensively by metalsmiths of the Kiowa and Pawnee tribes in Oklahoma.
Nickel silver looks much like true silver, although it can have a slight goldish tone to it. It is highly tarnish resistant and will only slightly darken with age - it really doesn't react to liver of sulfur, unlike sterling silver and copper relatives such as bronze, brass, and pure copper. Nickel silver is especially in chainmaille, where it is prized for its affordability and tarnish resistance. Could you imagine polishing a large bracelet made from sterling silver? How about an entire garment!
Nickel silver was developed, as an alternative to the Chinese alloy paktong, by German metalworkers in the 19th century. The Chinese developed this silver look-alike centuries ago, with secret exports to Southeast Asia resulting in an effort by colonizing Europeans to duplicate the guarded formula. In the 1700s metalworkers got close, and in 1823, German competition yielded a winner. Around the same time, a British man discovered a similar metal alloy.
Following its discovery, the use of nickel silver exploded. It has been used in on dining tables nearly since its discovery; some sets of silverware are stamped EPNS, which stands for Electro-plated nickel silver, which is silverware that has been made with nickel silver and electroplated with real silver. These "silver" sets are of no value to a metal recycler, but are becoming more rare every year, so perhaps they will have historical value. Also, as the silver finish wears away, the nickel silver is actually brighter, due to its tarnish resistance, so an EPNS set Used practically everywhere: keys, zippers, jewelry, silverware, wind instruments including French horns and flutes (can be silver-plated) and frets of guitars, automobiles, coins and even tracks in model railroads. It typically resists tarnish and corrosion. Used extensively by metalsmiths of the Kiowa and Pawnee tribes in Oklahoma.
Nickel silver looks much like true silver, although it can have a slight goldish tone to it. It is highly tarnish resistant and will only slightly darken with age - it really doesn't react to liver of sulfur, unlike sterling silver and copper relatives such as bronze, brass, and pure copper. Nickel silver is especially in chainmaille, where it is prized for its affordability and tarnish resistance. Could you imagine polishing a large bracelet made from sterling silver? How about an entire garment!
A NOTE ABOUT ALUMINUM
I should briefly mention aluminum: while this metal can look similar to silver, it can lose its shine and become dull. While some people enjoy using aluminum jump rings in chainmaille (it is very light, and very inexpensive), for traditional wire jewelry uses such as bundle bracelets, cabochon pendants, and prong rings, aluminum is too soft to stay in place, and is very difficult to work-harden. Although you can find aluminum wire online, we are proud to provide you with the 3 most-loved base metals - brass, copper, and nickel silver - in the gauges and shapes wire artists love.
I hope you've enjoyed these articles about base metal wire - Happy Jewelry Making!
I hope you've enjoyed these articles about base metal wire - Happy Jewelry Making!
BRONZE AND BRASS
Both bronze and brass are alloys of copper. The amount of copper and the other metals that it is mixed with are what determines the color and properties of the resulting metal.
The difference between bronze and brass, to many people, is irrelevant or not worth distinguishing. Since metal content varies throughout history, many museums simply describe possible bronze or brass artifacts as "copper alloy" pieces. However, in the jewelry world, the traditional terms still have important meaning. Another place where the distinction is important is the world of coin collecting, which has its own rich history of the metals and tokens used to create currency through time - a fascinating subject for another day, perhaps!
In the jewelry world, bronze is traditionally a warm, tan gold color, and will gradually acquire a brownish patina. On the other hand, brass is a vibrant color, and when it tarnishes, it develops the green verdigris seen on copper statues. Bronze is commonly patinaed and associated with Steampunk and Victorian jewelry, while brass is used in place of gold in many online jewelry storefronts.
The difference between bronze and brass, to many people, is irrelevant or not worth distinguishing. Since metal content varies throughout history, many museums simply describe possible bronze or brass artifacts as "copper alloy" pieces. However, in the jewelry world, the traditional terms still have important meaning. Another place where the distinction is important is the world of coin collecting, which has its own rich history of the metals and tokens used to create currency through time - a fascinating subject for another day, perhaps!
In the jewelry world, bronze is traditionally a warm, tan gold color, and will gradually acquire a brownish patina. On the other hand, brass is a vibrant color, and when it tarnishes, it develops the green verdigris seen on copper statues. Bronze is commonly patinaed and associated with Steampunk and Victorian jewelry, while brass is used in place of gold in many online jewelry storefronts.
BRASS
Brass, an alloy of copper and zinc, is a stronger metal than its parent copper, though not as strong as steel. Sometimes other metals such as lead and tin are added to make the brass more workable. By 300 AD, Germany and the Netherlands had become well-known for their brass. By 1852, brass cartridges were in production: the brass metal expanded as the gun was fired and contracted after firing, which allowed for the development of automatic weapons.
Brass comes in a couple of varieties - yellow and red. Red brass is an alloy somewhat between bronze and traditional brass. It contains more copper - a ratio of 85% copper to 15% zinc - which gives it its warm red color. Yellow, an alloy of roughly 67% copper to 33% zinc, produces an almost fluorescent yellowish hue that many people use in place of gold. You'll recognize yellow brass in several spots around your home - door hinges, for instance. One of yellow brass's great distinguishing traits is that it's shinier and brighter than red brass.
In home uses, such as taps and lamps, brass is typically lacquered to protect the brass. Therefore, when cleaning brass around the house, don't be abrasive, but use a polish and lightly buff it. You can use the same processes to protect your brass jewelry as you would silver or copper pieces: with wax, sealants, or sprays.
Brass comes in a couple of varieties - yellow and red. Red brass is an alloy somewhat between bronze and traditional brass. It contains more copper - a ratio of 85% copper to 15% zinc - which gives it its warm red color. Yellow, an alloy of roughly 67% copper to 33% zinc, produces an almost fluorescent yellowish hue that many people use in place of gold. You'll recognize yellow brass in several spots around your home - door hinges, for instance. One of yellow brass's great distinguishing traits is that it's shinier and brighter than red brass.
In home uses, such as taps and lamps, brass is typically lacquered to protect the brass. Therefore, when cleaning brass around the house, don't be abrasive, but use a polish and lightly buff it. You can use the same processes to protect your brass jewelry as you would silver or copper pieces: with wax, sealants, or sprays.
BRONZE
Bronze is an alloy of copper and tin, typically an 88% copper to 12% tin mix (although it doesn't always contain tin), and can contain elements such as manganese, silicone, aluminum, and phosphorous. Early bronze contained arsenic, which was replaced with tin, a nontoxic alternative.
Bronze is hard yet brittle, used in weapons, tools, and armor dating back to 3000 BC (one source I read noted that bronze swords were more for stabbing than slicing!). The Iron Age replaced the Bronze Age, but wrought iron is actually weaker than bronze; the big difference is that iron was more economical, so it replaced bronze everywhere strength was needed. Tin was particularly hard to find, which is why bronze fell out of favor. (After Iron came Steel).
Bronze is hard yet brittle, used in weapons, tools, and armor dating back to 3000 BC (one source I read noted that bronze swords were more for stabbing than slicing!). The Iron Age replaced the Bronze Age, but wrought iron is actually weaker than bronze; the big difference is that iron was more economical, so it replaced bronze everywhere strength was needed. Tin was particularly hard to find, which is why bronze fell out of favor. (After Iron came Steel).
ORMOLU AND DORE
As a point of interest: we already know that when a coat of gold is applied to silver, the result is called vermeil, also called gold vermeil. When gold is applied to bronze, it is called ormolu (or gilt bronze). When gold is applied to brass, it is called doré (or gild brass). However, this is a rare effect to find nowadays, because it required the use of mercury. The mercury-firing process was outlawed by France in the 19th century due to the grievous toll on gilders' health. However, some locations continued the mercury process, producing gilt bronze as late as 1960. The gilt brass and gilt bronze effect was extremely popular for jewelry, chandeliers, clocks, candelabras, ceramics, and sculptures in the Rococo and Neoclassical periods in Europe and in some Chinese areas as well. In fact, there was even some ormolu work on the Grand Staircase of the Titanic, which was decorated to recall the culture of King Louis XIV.
The allure of ormolu was that as time weathered the metal, the gold would not tarnish or fade (excellent in households). It also served as a contrast to nearby raw bronze (such as in sculptures - just the hair would be gilded, for example). To care for gilded brass, gilded bronze, or vermeil for that matter, avoid the polishing cloth. Simply wash with mild soap and water to clean the piece and reduce the risk of flaking or rubbing the gilded layer off.
The allure of ormolu was that as time weathered the metal, the gold would not tarnish or fade (excellent in households). It also served as a contrast to nearby raw bronze (such as in sculptures - just the hair would be gilded, for example). To care for gilded brass, gilded bronze, or vermeil for that matter, avoid the polishing cloth. Simply wash with mild soap and water to clean the piece and reduce the risk of flaking or rubbing the gilded layer off.
Materials

Copper Wire

Red Brass Wire

Yellow Brass Wire

Nickle Silver Wire

Copper Sheet

Red Brass Sheet

Nickle Sheet
Tools

WireJewelry - Ultimate Wire-Pliers Jewelry Pliers with Case, Set of 5
G15-20
- G15-20
- Lesson Quantity: 1.00 pieces
- Purchase Quantity: 1.00 each
- Price: $170.72
- Gold Club Price: $128.04

Bench Tools
- Category: General Education
- Technique(s): Oxidizing / Antiquing, General Education





















 About Jewelry Chain- About Ball Chain
About Jewelry Chain- About Ball Chain About Jewelry Chain- Snake Chain and Omega Chain
About Jewelry Chain- Snake Chain and Omega Chain About Jewelry Chain- Bar Chain and Peanut Chain
About Jewelry Chain- Bar Chain and Peanut Chain About Jewelry Chain - Cable Chain and Rolo Chain
About Jewelry Chain - Cable Chain and Rolo Chain About Jewelry Chain- Curb Chain and Gourmette Chain
About Jewelry Chain- Curb Chain and Gourmette Chain About Jewelry Chain- Figaro Chain
About Jewelry Chain- Figaro Chain About Jewelry Chain- Infinity Chain and Anchor Chain
About Jewelry Chain- Infinity Chain and Anchor Chain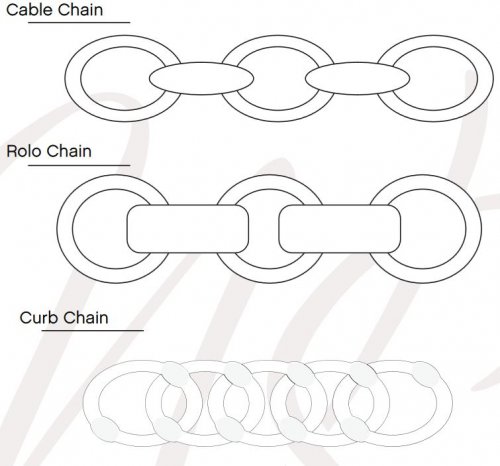 About Jewelry Chain- Chain Reference Sheet
About Jewelry Chain- Chain Reference Sheet About Jewelry Chain- Venetian Chain and Box Chain
About Jewelry Chain- Venetian Chain and Box Chain About Jewelry Chain- Wheat Chain and Rope Chain
About Jewelry Chain- Wheat Chain and Rope Chain Introduction to Chain
Introduction to Chain Access More Money by Making Jewelry When Your Prices Are Right
Access More Money by Making Jewelry When Your Prices Are Right An Introduction to Beads and Beading
An Introduction to Beads and Beading Common Gemstone Misconceptions
Common Gemstone Misconceptions Wire Wrapped Christmas Tree
Wire Wrapped Christmas Tree How To Polish Metal Jewelry using a Rotary Tumbler
How To Polish Metal Jewelry using a Rotary Tumbler How To Polish Your Own Rocks using a Rotary Rock Tumbler
How To Polish Your Own Rocks using a Rotary Rock Tumbler How to Merchandise Your Jewelry on the Internet
How to Merchandise Your Jewelry on the Internet How to Use Twitter as a Wire Jewelry Artist
How to Use Twitter as a Wire Jewelry Artist 20 Ideas to get your Jewelry Biz Busy
20 Ideas to get your Jewelry Biz Busy Watching the Precious Metals Market
Watching the Precious Metals Market Jewelry Design Ideas - Get Inspired
Jewelry Design Ideas - Get Inspired Measuring Tools
Measuring Tools July Birthstone - The Ruby
July Birthstone - The Ruby February Birthstone- Amethyst
February Birthstone- Amethyst March Birthstone - Aquamarine and Bloodstone
March Birthstone - Aquamarine and Bloodstone September Birthstone - Sapphire
September Birthstone - Sapphire November Birthstones - Topaz and Citrine
November Birthstones - Topaz and Citrine October Birthstones - Rose Zircon, Pink Tourmaline and Opal
October Birthstones - Rose Zircon, Pink Tourmaline and Opal April Birthstone - The Diamond
April Birthstone - The Diamond August Birthstone - Peridot and Sardonyx
August Birthstone - Peridot and Sardonyx June Birthstones - Alexandrite, Pearl and Moonstone
June Birthstones - Alexandrite, Pearl and Moonstone Metalsmithing
Metalsmithing Featured Tool - Mini TruStrike Hammers
Featured Tool - Mini TruStrike Hammers Natural Jasper Stones - Cabochon Gemstones
Natural Jasper Stones - Cabochon Gemstones Organize Your Jewelry Box
Organize Your Jewelry Box Pearls- It's a Cultural Thing
Pearls- It's a Cultural Thing Soldering 101
Soldering 101 Starting Your Own Home Jewelry Business
Starting Your Own Home Jewelry Business The Art of Creating Chainmail
The Art of Creating Chainmail Why Should I Be Using Facebook
Why Should I Be Using Facebook Make Handmade Neck Cords on a Dime
Make Handmade Neck Cords on a Dime Tagging Handmade Jewelry Gifts
Tagging Handmade Jewelry Gifts Share Your Expertise with Your Community
Share Your Expertise with Your Community Creating Color Schemes for Jewelry Making
Creating Color Schemes for Jewelry Making Gemstone Treatments
Gemstone Treatments How Wire is Made
How Wire is Made Beading A-B-C's
Beading A-B-C's How to Set Up Your Workspace
How to Set Up Your Workspace Gem Profile- Diamond
Gem Profile- Diamond Gem Profile- Peridot
Gem Profile- Peridot Gem Profile- Goldstone
Gem Profile- Goldstone Gem Profile- Cryptocrystalline Quartz Introduction
Gem Profile- Cryptocrystalline Quartz Introduction Gem Profile- Banded Agate and Brecciated Agate
Gem Profile- Banded Agate and Brecciated Agate Gem Profile- Emerald
Gem Profile- Emerald Gem Profile- Titanite or Sphene
Gem Profile- Titanite or Sphene Gem Profile- Morganite
Gem Profile- Morganite Gem Profile- Desert Rose
Gem Profile- Desert Rose Gem Profile- Iolite
Gem Profile- Iolite Gem Profile- Zultanite
Gem Profile- Zultanite Gem Profile- Maw Sit Sit
Gem Profile- Maw Sit Sit Gem Profile- Tanzanite
Gem Profile- Tanzanite Gem Profile- Aquamarine
Gem Profile- Aquamarine Gem Profile- Turquoise
Gem Profile- Turquoise Gem Profile- Turquoise Types
Gem Profile- Turquoise Types Gem Profile- What's Druze
Gem Profile- What's Druze Gem Profile- Basalt
Gem Profile- Basalt Gem Profile- Fordite
Gem Profile- Fordite Gem Profile- Variscite
Gem Profile- Variscite Gem Profile- Pearls
Gem Profile- Pearls Gem Profile- Onyx
Gem Profile- Onyx Gem Profile- Sunstone
Gem Profile- Sunstone Gem Profile- Sonora Sunrise
Gem Profile- Sonora Sunrise Gem Profile- Rhodonite
Gem Profile- Rhodonite Gem Profile- Glass, Crystal and Quartz
Gem Profile- Glass, Crystal and Quartz Gem Profile- Psilomelane
Gem Profile- Psilomelane Gem Profile- Fulgurite
Gem Profile- Fulgurite Gem Profile- Cat's Eye
Gem Profile- Cat's Eye Gem Profile- Carnelian
Gem Profile- Carnelian Gem Profile- Petoskey Stones and Indonesian Fossil Coral
Gem Profile- Petoskey Stones and Indonesian Fossil Coral Gem Profile- Rutilated Quartz
Gem Profile- Rutilated Quartz Gem Profile- Chrysocolla
Gem Profile- Chrysocolla Gem Profile- Jet
Gem Profile- Jet Gem Profile- Chrysoprase
Gem Profile- Chrysoprase Gem Profile- Rhyolite
Gem Profile- Rhyolite Gem Profile- Chalcedony
Gem Profile- Chalcedony Gem Profile- Lepidolite and Sugilite
Gem Profile- Lepidolite and Sugilite Gem Profile- Unakite
Gem Profile- Unakite Gem Profile- Cowrie Shells, Conch Shells, and Drilling Shells
Gem Profile- Cowrie Shells, Conch Shells, and Drilling Shells Gem Profile- Mother of Pearl
Gem Profile- Mother of Pearl Gem Profile- Moss Agate and Plume Agate
Gem Profile- Moss Agate and Plume Agate Gem Profile- Thundereggs and Mexican Lace Agate
Gem Profile- Thundereggs and Mexican Lace Agate Gem Profile- Dumortierite
Gem Profile- Dumortierite Gem Profile- Apatite
Gem Profile- Apatite Gem Profile- Blue Topaz
Gem Profile- Blue Topaz Gem Profile- Aragonite
Gem Profile- Aragonite Gem Profile- Zircon and Cubic Zirconia
Gem Profile- Zircon and Cubic Zirconia Gem Profile- Topaz
Gem Profile- Topaz Gem Profile- Howlite
Gem Profile- Howlite Gem Profile- Sodalite
Gem Profile- Sodalite Gem Profile- Magnesite
Gem Profile- Magnesite Gem Profile- Cuprite
Gem Profile- Cuprite Gem Profile- Nuummite
Gem Profile- Nuummite Gem Profile- Bronzite
Gem Profile- Bronzite Gem Profile- Kyanite
Gem Profile- Kyanite Gem Profile- Hematite
Gem Profile- Hematite Gem Profile- Derbyshire Blue John
Gem Profile- Derbyshire Blue John Gem Profile- Eilat Stone
Gem Profile- Eilat Stone Gem Profile- Vesuvianite
Gem Profile- Vesuvianite Gem Profile- Strontium Titanate -Fabulite
Gem Profile- Strontium Titanate -Fabulite Gem Profile- Tourmaline
Gem Profile- Tourmaline Gem Profile- Larimar
Gem Profile- Larimar Gem Profile- Garnet
Gem Profile- Garnet Gem Profile- Tsavorite and Green Garnets
Gem Profile- Tsavorite and Green Garnets Gem Profile- Seraphinite
Gem Profile- Seraphinite Gem Profile- Serpentine
Gem Profile- Serpentine American Wire Gauge
American Wire Gauge Viking Knit and Spool Knit Chain
Viking Knit and Spool Knit Chain Copper Roses
Copper Roses How to Make Medical ID Bracelets Special
How to Make Medical ID Bracelets Special Remembering the Fallen
Remembering the Fallen 6 Ways to Find Your Uniqueness in Jewelry
6 Ways to Find Your Uniqueness in Jewelry Gem Profile- Moissanite
Gem Profile- Moissanite Birthstone Swarovski Colors
Birthstone Swarovski Colors Gem profile- Paua and Abalone
Gem profile- Paua and Abalone Tips for Tucson Shopping- Gem Show Secrets
Tips for Tucson Shopping- Gem Show Secrets Durston Olivia Rolling Mills
Durston Olivia Rolling Mills How to Use a Jewelry Bench Polisher Effectively
How to Use a Jewelry Bench Polisher Effectively 
